Ringing in the New Year!

Welcome 2023! But before we leave 2022 behind, this end-of-year newsletter features a big OBI project that wrapped up in 2022, plus a recap of some of the outstanding and innovative projects that our client companies are working on. We hope you enjoy sharing in their success stories as much as we do.
This special edition of the OBI & OTRADI newsletter also includes some helpful information for our readers, including updates, opportunities, and some really great upcoming events. Please feel free to share. We hope you have a peaceful and prosperous 2023.
Our HIOP Space is Completed:
Thank you to Business Oregon!

In 2021, OBI/OTRADI was granted High Impact Opportunity Project (HIOP) implementation funding from Business Oregon to build out new space to support growth of the bioscience sector in Oregon. We got right to work, transforming our prior administrative offices in the Willamette Wharf building into new labs and offices. Due to supply chain issues during a global pandemic, progress was not always speedy, but this project is now fully complete. Thank you, Business Oregon!
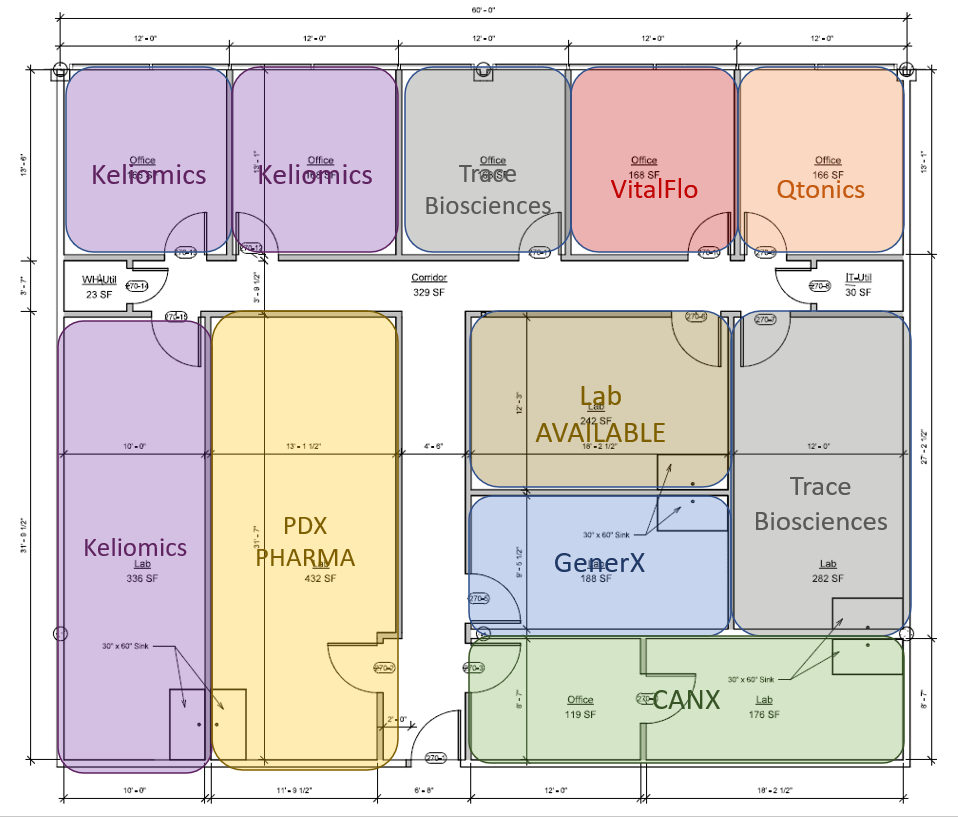
The OBI used the HIOP dollars to fund the build-out of 3,300 square feet into six labs and six offices to expand incubator space for startup bio companies. This new space funded via HIOP dollars has allowed us to bring in seven new companies from our waiting list into the OBI facility. One additional lab is still unleased and available to one of OBI’s current companies or a new company upon completion of our selection committee process. For life science startups seeking space, inquire here.

The seven companies in the new space include CANX, GenerX, Keliomics, PDX Pharmaceuticals, Qtonics, Trace Biosciences (formerly Inherent Targeting), and VitalFlo; they support 17 jobs with one company actively hiring an additional position. These companies have raised over $7,527,000 in funding, which was mainly secured via SBIR grants and seed funding.
Client Company Wins in 2022
Aronora (alumnus)
- Aronora co-founder and CEO Dr. Erik Tucker has been elected as Fellow of the National Academy of Inventors (NAI).
- Aronora Announces Clinical Data to Be Presented at the 64th American Society of Hematology Annual Meeting. Presentation to include clinical data from a phase 2 study of Gruticibart (AB023), a factor XI-targeted antibody being developed for the treatment and prevention of thrombosis and inflammation.
- CANX, LLC joined the OBI in October 2022
- CANX is developing therapeutics to address iron-related health conditions such as iron overload disease and certain forms of cancer which depend on iron for their growth.
- Founder Kerry Masterson successfully completed the OHSU I-Corp 5-week BIP Corps program.
Caregiven (alumnus)
- In our latest product update we launched our affiliate program with 4 amazing companies who support caregivers: Grief Coach, SilverBills, eFuneral, and Pinventory. While Caregiven does not advertise to our users, we do offer referral links to our affiliate partners who offer users a discount and provide Caregiven with lead generation revenue.
- Alaska’s Dementia Action Collaborative profiled Caregiven as a valuable resource to its stakeholder community in their recent newsletter.
- Caregiven was selected to participate in the APIS Health Angel program.
- Caregiven was 1 of 4 finalists from the Australian round of the Zurich Innovation Championship. Selected from an applicant pool of over 90 insuretech solutions, Caregiven presented to Zurich’s Australian executive team including the global head of their LifeWell initiative.
- We also surpassed 1,000 sign-ups in mid-February – that’s over one thousand families going through a caregiving event globally who are benefitting from our product.
- Founder and CEO Candice Smith was a featured panelist at OBI’s February Lunch & Learn on How Accelerators Can Help Boost Your Startup.
- Caregiven was acquired by Delta Dental of Iowa and its subsidiary Veratrus Health. Candice Smith, Caregiven’s founder and CEO, has joined the Delta Dental team! Read the press release here.
- CytoImage was featured in the OHSU Innovates 2022 Impact Report.
- EndoSound is pleased to announce two new full-time employees. We are thrilled to have these two join our growing team.
- Peter Hoffman has joined as Senior Product Development Engineer. He will help to bring new and innovative ultrasound products to market. His 10+ years engineering experience ranges from robotics to medical devices with much of his time at Olympus developing novel biopsy tools for the lungs. He has authored 6 patent applications. Peter holds a BS in Mechanical Engineering and a minor in Business and Entrepreneurship from Oregon State University.
- Patrick Hurley has joined as the Vice President of Marketing. Patrick comes with 13 years in product and market management in medical device, as well as several years as a life sciences consultant. Patrick has documented successes in each role as he continued to grow with responsibilities. He has helped build brand identity and established strong relationships with KOLs in the GI community. He holds a Ph.D. in biology, specializing in neuroscience from Wesleyan University.
- Winner of AGA Shark Tank and Innovation of the year. We had the opportunity to compete in the American Gastroenterology Association (AGA) Shark Tank for innovative technologies in early April and won!!
- We’ll be competing again with our innovative Endoscopic Ultrasound device at the end of May at the largest Gastroenterology professional conference in the world called Digestive Disease Week (DDW).
- We have also been honored with the Innovation of the Year from the American Society for Gastrointestinal Endoscopy (ASGE). This distinction is granted to companies with products that show promise for advancing the field of GI endoscopy.
- Together, these awards demonstrate to the broader GI community that our mission to democratize EUS is taking shape. There could not be a better time to follow the EndoSound journey. View our LinkedIn page.
- We recently announced Scott Fraser as a new addition to our board. Scott comes with a world of knowledge in startups, the Gastroenterology (GI) space, and the Ambulatory Surgical Center (ASC) market.
- In late May, we had our first ever booth at Digestive Disease Week (DDW). This conference is the premier conference for the GI space, attracting doctors, industry professionals, and investors from all over the world. We had tremendous response at the booth and as a result of several meetings with physicians and strategic partners.
- Some of the Endosound team, in partnership with physicians in Ecuador, showcased the Endosound Vision System (EVS) on Endoscopy on Air (EOA) on June 3rd. The Global Annual Live Streaming Event on EOA had physicians from around the world educate and demonstrate their techniques in various endoscopic procedures to the 11,000 virtual attendees. We were honored to sponsor a session with partner Dr. Carlos Robles Medranda at the Ecuadorian Institute of Digestive Disease.
- Our podcast with Expert Dojo was released online.
- MedRxiv published our study of gamma prime fibrinogen in COVID-19 patients.
- David Farrell, Founder and CSO participated as a featured panelist at OBI’s February Lunch & Learn on How Accelerators Can Help Boost Your Startup.
- Gamma Diagnostics recently participated in the Expert Dojo international accelerator program.
- We are thrilled to announce the promotion of Jamie Noll, Pharm.D., B.S. Nutrition to Chief Scientific Officer for Gamma Diagnostics Inc., effective immediately. Jamie joined the Gamma Dx team late last year as a scientific and clinical advisor and in a very short time has helped establish key KOL relationships in the medical and clinical research community. She has had various roles and extensive experience in the pharmaceutical industry last of which was as a Medical Science Liaison for Puma Biotechnology.
- “I have known Jamie for over 14 years and our professional and personal relationship has been forged through challenging projects and arduous decisions. Not only is she extremely talented in her field but she represents the highest standard in ethics and integrity. I feel honored and privileged she has decided to join us in the CSO capacity.” Ajay Nair, CEO Gamma Diagnostics Inc.
- “Dr. Noll is a fabulous addition to the Gamma Diagnostics team! Her industry experience and scientific expertise are critical assets that will help propel Gamma Diagnostics forward.” Dr. David Farrell, Founder, Scientific Advisor, Gamma Diagnostics Inc.
- Gamma Diagnostics presented at the Expert Dojo Investor Festival on April 7 in Los Angeles. Our new CSO, Dr. Jamie Noll, and Founder Dr. David Farrell presented. See event details here.
- We filed a new provisional patent: Provisional Patent Application in United States No. 63/268,605 ‘Use of γ’ fibrinogen as a Biomarker in the Detection of Covid-19 and Prognosis of Severe Disease’
- We filed an Emergency Use Authorization to the FDA entitled: “Gamma-prime fibrinogen ELISA”
- Gamma Diagnostics Inc. and OHSU have signed a licensing agreement that seeks to further develop and commercially promote the use of Gamma Prime Fibrinogen (GPF) as an inflammatory and clotting risk biomarker.
- High GPF levels have previously shown positive correlation with peripheral artery disease, heart failure and CVD deaths in the ARIC study. Dr. David Farrell (Founder and CSO) published an abstract at AHA 2021 showing COVID-19 patients can develop extraordinarily high levels of GPF.
- Gamma Diagnostics Inc. will now continue to invest in further clinical studies, regulatory submission, and commercialization activities to bring GPF as a biomarker to market.
- Gamma Diagnostics Inc. is proud to announce that Mr. Ajay Nair, currently President and CEO, will serve as Chairman of the Executive Board and transition the Chief Executive Officer role to Mr. Chris Venter, currently CFO of the Company, effective November 18th, 2022.
- GenerX moved its headquarters from Santa Clara, CA into one of the brand news labs in the OBI in Portland on December 1, 2022.
- GenerX is a pharmaceutical development company, has moved its headquarters from California to the OBI. GenerX is engaged in the development, manufacturing, acquisition, licensing, and partnership of sterile injectable generic drug products for the U.S. and overseas markets. Their mission is to provide affordable generic medicines to patients through innovative solutions by leveraging our expertise and partnerships with world class FDA approved manufacturing.
- Hemex Health pitched to Inflect Health in January 2022.
- Whitney Wilhardt has joined Hemex as PR and Marketing Specialist.
- We sent our first Gazelles to Riyadh in Saudi Arabia.
- We’re scheduled to ship commercially to Bahrein, Oman, Kuwait and Algeria, and we are already selling to Lebanon and UAE (Dubai and Abu Dhabi).
- We’ve partnered with distributors in Savona, Italy, our first European location.
- Umut Gurkan, our Chief Scientist for Sickle Cell Disease, based at Case Western in Ohio, was elected into the American Institute for Medical and Biological Engineering’s College of Fellows for his continuing achievements in medical and biological engineering.
- Patti White, Co-founder and CEO, recently participated as a presenter in an Angel Oregon Bio educational seminar and was be a featured panelist in two of OBI’s Lunch & Learns on Women in Leadership. View the March event here and the June event here.
- We received the 2022 West Coast Consortium for Technology & Innovation in Pediatrics (CTIP) grant award for catalyzing pediatric innovation (CPI), which supports the development of pediatric innovation from ideation through commercialization. We plan to use this grant to help apply for US FDA clearance to bring our affordable POC sickle cell test to the US.
- We have two abstracts that were accepted for the 4th Global Congress on Sickle Cell Disease (June 16-18) in Paris.
- “Point-of-care diagnostic for quantification of fetal haemoglobin (Hb F) levels in monitoring hydroxyurea therapy for children with Sickle Cell Disease (SCD) in Ghana” has been accepted for a poster presentation.
- “Evaluation of first microchip-based point-of-care device for diagnosis of Beta-Thalassemia in India” has been accepted for a poster presentation.
- Hemex Health announced a significant enhancement to their Gazelle™ Hb Variant test, which allows for more precise measurements of Hb F, also known as fetal hemoglobin. The increased accuracy could be useful for monitoring hydroxyurea therapy in point-of-care settings. Hemex will provide “over the air” updates with the Hb F enhancement that can be downloaded directly to existing Gazelle Readers. The improvement will ship with new systems.
- We are proud to announce the official launch of the Gazelle™ PathoCatch™ COVID-19 FIA test, a diagnostic solution for point-of-care testing, made in collaboration with @MylabSolutions. Read the full press release.
- We received National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for continuing to support Hemex Health and Case School of Engineering at Case Western Reserve University with a $1.7M Phase II STTR follow up award for our “portable, affordable, quantitative microchip electrophoresis for hemoglobin A1C (diabetes) testing.”
- We launched our COVID-19 point-of-care diagnostic, Gazelle PathoCatch, in India on July 7 and plan to launch to our other distributors worldwide later this summer.
- CEO and Co-Founder Patti White was interviewed by International Finance Corporation (IFC).
- We received Portland Business Journal’s Global Impact Award as part of their Health Care of the Future Program. Patti White, CEO and co-founder, accepted the award during their ceremony held on August 18.
- Gazelle received Indian Council of Medical Research (ICMR) recommendation for sickle cell testing in India. Gazelle was one of three diagnostic platforms selected for this highly competitive and rigorous nod of approval.
- We are proud to announce during this Sickle Cell Awareness Month (September 2022) that Hemex has shipped 210,000 SCD tests to 25 countries.
- Hemex presented two posters at the Tropical Medicine Show (ASTMH) in Seattle.
- The first discussed research on Gazelle’s ability to detect and differentiate P. knowlesi (a malaria species indigenous to SE Asia) from other malaria species.
- The seconds was a study conducted in Ghana on Gazelle’s ability to measure fetal hemoglobin, which has possible applications for monitoring treatment for sickle cell disease.
- NIH Seed sponsored Hemex at the Redefining Early-Stage Investments (RESI) Conference, January 10, 2023, which connects startups with early-stage investors.
- The Zambian Network for Sickle Cell Disease in conjunction with the Zambian Ministry of Health launched Gazelle SCD testing devices in several district hospitals.
- Hemex presented a poster on our beta thalassemia test at the American Society of Hematology (ASH) in New Orleans in December 2022.
- Health Technology Innovations, Inc. was awarded a $1 million SBIR Phase II grant from the National Science Foundation to commercialize CryoDiscovery ™, a Cryo-EM Automation and Intelligence Platform for Drug Discovery.
- HTI is hiring AI/ML software development engineers, interns & post docs.
- Health Technology Innovations, Inc. (HTI) is excited to announce that Dr. Doug Kawahara is joining HTI as Senior Advisor for AI/ML in Drug Discovery. Dr. Kawahara brings extensive experience in drug discovery and development to HTI. He is currently President & CEO of Elex Biotech (Portland, OR), a company developing novel drugs for the treatment of arrhythmia and heart failure. Read full press release.
- HTI attended the 17th Annual Drug Discovery Chemistry event in San Diego on April 18-22, presenting a Poster session on CryoDiscovery™ its machine learning platform for cryo-EM image processing, and providing information on its cryo-EM Services offerings for drug discovery.
- HTI CTO, Narasimha Kumar, presented a poster session virtually at The Astbury Conversation 2022, on April 25-26 on CryoDiscovery™: Public Data-based AI/ML model enhancements for Cryo-EM Image Analysis. The Astbury is one of Europe’s premier conferences on macromolecular research, including a keynote by cryo-EM Nobel Laureate Richard Henderson.
- HTI has signed master service agreements with IDEAYA Biosciences and ADRx for cryo-EM structure determination services to support their drug discovery efforts. IDEAYA is a synthetic lethality-focused precision medicine oncology company and ADRx is a biotechnology company with a unique approach to halting diseases of protein aggregation.
- Received Phase II matching grant award from Business Oregon.
- In collaboration with Oregon BIO, HTI attended the Bio International 2022 with great success.
- HTI presented CryoDiscovery™ and CryoFast™ at the Microscopy & Microanalysis 2022 and American Crystallographic Association 2022 conferences on July 29 – Aug 4 in Portland, Oregon
- HTI signed a service agreement with ADRx Pharma for Cryo-EM sample optimization and image analysis.
- Signed Master Service Agreement with ADRx and a Top Tier International Pharmaceutical company for Cryo-EM services.
- HTI is now offering its Cryo-EM services via Scientist.com, a leading “science as a service” e-commerce site for Life Sciences. This adds a second e-commerce sales channel beyond ScienceExchange.com, the world’s first online R&D marketplace to accelerate scientific discovery, of which HTI became a member last year.
- Set up collaboration with Salk Institute for Cryo-EM data sets sharing.
- Filed patent of CryoFAST™ architecture (CRYOGENIC ELECTRON MICROSCOPY FULLY AUTOMATED ACQUISITION FOR SINGLE PARTICLE TOMOGRAPHY).
- Signed Master Service Agreement with AstraZeneca for Cryo-EM single particle analysis.
- Delivered Cryo-EM 3D structure model with encouraging results for ADRx.
- Jared Lynch has been made Sales Director for the United States region as of October 2022.
- Anthony McNeil is now the West Coast Sales and Applications Scientist.
- Izon will complete our Gen 2 qEV column releases with all sized columns available after January 17, 2023. This includes qEV singles, originals, 1s, 2s, 10s, and 100s.
- Izon is quite pleased with the adoption of our version 2 of the Automatic Fraction Collector which was released in February 2022.
- We have several new product releases planned for 2023.
- We are excited for ISEV2023 in Seattle in May, where Izon will be a sponsor.
- Joined the OBI in October 2022. They moved into two of OBI’s new offices and one of the brand-new labs.
- Keliomics offers pharmacophenotyping for patients with solid tumors including breast, prostate, and certain types of metastatic cancer.
- Keliomics was one of the five winners of the Beaverton Startup Challenge 2023, winning $25,000.
- We were selected for the inaugural class of Series P– a collaboration accelerator sponsored by Procter & Gamble Ventures and Plug and Play.
- We participated on a FemTech panel along with Convey Capital hosted by Stanford Biodesign.
- Holly graduated from the Ferolyn Fellowship program.
- CEO Holly Rockweiler was a presenter at a recent Angel Oregon Bio educational seminar.
- In February, Holly was featured as UCSF’s Rosenman Healthtech leader of the month.
- Madorra was featured on the Medsider podcast in March.
- We were selected out of a record-setting number of applicants to present at the McKinsey Early Stage Investor Conference in June.
- We were featured on a Washington University in St. Louis panel entitled: WashU Women Changing the Way We Live and Work.
- We were selected (for the second time!) as one of the showcase companies for the NIH SBIR Investor Initiatives program. The NIH will be sponsoring us to attend the AdvaMed conference in Boston, MA this fall.
- NeuvaRx joined the OBI in March 2022. NeuvaRx is preclinical-stage biopharmaceutical company dedicated to the development of therapies and diagnostic tools for neurovascular disorders, including, but not limited to ischemic and hemorrhagic stroke and vascular dementia.
- NeuvaRx received an NIH phase I STTR award that started July 1.
- We also received a matching grant award from Oregon Business Development for the STTR award.
- OmnEcoil was mentioned in a May 2022 report entitled: “Global Biopsy Devices Pipeline Market Landscape Report 2021: Stage of Development, Segment, Regulatory Path, Estimated Approval Date, Ongoing Clinical Trial – ResearchAndMarkets.com.” You can read the press release here.
- PDX Pharmaceuticals joined the OBI in February 2022. PDX Pharmaceuticals is an IND-enabling stage biopharmaceutical company bringing complementary biological pathways together to achieve synergistic clinical benefits. Their patented mesoporous silica-based nanoparticle platform (Pdx-NP) has been optimized to co-deliver multiple classes of therapeutics (e.g., siRNA, small molecule drugs, antibodies, immunostimulants) to ensure that they reach the target cells at the same time to fully realize their synergy. It can deliver cargos to both cancer and immune cells. Their current focus is to generate effective anti-tumor immune response through profound CD8+ anti-tumor T cell production.
- We have a new publication in Nature Communications.
- Moataz Reda, Worapol Ngamcherdtrakul, Molly A Nelson, Natnaree Siriwon, Ruijie Wang, Husam Y Zaidan, Daniel S Bejan, Sherif Reda, Ngoc Ha Hoang, Noah A Crumrine, Justin PC Rehwaldt, Akash Bindal, Gordon B Mills, Joe W Gray, Wassana Yantasee. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nature communications. 13(1), 1-11, 2022.
- Also we won a matching grant for phase II small business innovative research (SBIR) from Business Oregon this year. The award (USD 100,000) will be used for our IP protection, financing a new facility, and fundraising activities.
- PDX Pharma was recognized by Portland Business Journal and received two philanthropic awards on October 27.
- 2022 Innovation in Philanthropy award (PDX Pharmaceuticals in partnership with OHSU).
- Top 10 philanthropic small companies (below $10M in revenue).
- These awards recognized our commitment, through donation and volunteer work, to support education and training in cancer research and nanotechnology.
- PDX Pharmaceuticals expanded within the OBI to an additional lab, one of the brand-new labs recently opened in Suite 270.
- PDX Pharma’s ARAC-02 drug candidate has been selected into the Nanotechnology Characterization Lab (NCL)’s collaboration and characterization program. ARAC-02 targets PD-L1, PLK1, and TLR9 for the treatment of lung cancer. More information here.
- Founders Dr. David Sheridan and Dr. Matthew Hansen co-authored a journal article in Frontiers in Medicine, entitled “Point-Of-Care Capillary Refill Technology Improves Accuracy of Peripheral Perfusion Assessment.”
- We are really excited to announce that Promedix has been selected into the highly competitive Creative Destruction Labs–Healthcare/Biomed Engineering Stream based out of Vancouver BC. This brings together investors, mentors, and advisors across all aspects of the product life cycle to accelerate commercialization.
- ProMedix was named as a semi-finalist for the Bend Venture Conference.
- ProMedix currently has an open round for funding and would love to discuss further with any interested parties at OBI or beyond. Please email Scott Filer, CEO: scott_filer@promedixinc.com.
- ProMedix was recently selected from a competitive group of startups for the Creative Destruction Lab‘s healthcare cohort. This will help to solidify a go-to-market strategy for our technology as well as expand to a large network of healthcare innovators.
- ProMedix has been announced as a finalist for the Seattle Angel Conference. In addition it is being highlighted as stage presenting startup company at the large Octane MedTech Innovation Forum in Southern California.
- We also brought onboard Robert Jenks, MBA as our Acting CFO. Bob is a former Managing Director for GE Equity.
- ProMedix was featured in the OHSU Innovates 2022 Impact Report.
- Qtonics moved within the OBI from a co-working desk space to one of our newly built offices.
- Launched a new product line specifically designed for gene therapy applications, called the SamuxMP. The Refeyn SamuxMP is a mass photometer optimized for adeno-associated virus (AAV) characterization and is an essential analytical tool for laboratories working with AAVs. The SamuxMP precisely measures the empty/full capsid ratio for AAVs of any serotype. SamuxMP mass photometry measurements are rapid and require very little sample.
- Brenda Watt has been promoted to Customer Success Manager in the Portland office, and Ritu is a new member of the Portland team.
- Refeyn closed Series B funding round, led by Northpond Ventures! Read their press release here.
- Received a Business Oregon grant award to develop a marketing strategy and hire additional staff for their AI-as-a-service platform in diagnostics and analysis.
- Rewire Neuro was a finalist in Pitch Oregon by TiE Oregon, in the Life Science category.
- John Harkness, President and CEO, was a presenter in an Angel Oregon Bio educational seminar.
- Rewire attended BIO International as part of the Oregon Pavilion and pitched at the Startup Stadium as a finalist, gaining recognition, feedback, and interest from a large group of investors and potential partners from around the world.
- Rewire Neuro, Inc., makers of Pipsqueak Pro – the revolutionary AI image analysis platform for biological researchers, announces their partner platform integration with eLabNext, part of Eppendorf Group, and premier Electronic Lab Notebook (ELN) platform provider.
- Rewire will be integrating our image analysis software into eLabNext’s electronic lab notebook, which will enable users to seamlessly move between conducting their image analysis and their collaborative data organizational environment, providing deeper analysis and insights for the entire lab.
- Pipsqueak Pro was born from the lab–developed by scientists to rapidly speed up manual image analysis, cellular quantification, and multi-channel (colocalization) analysis for both image and video media. The platform uses a patented machine learning system to improve your ROI predictions with minimal user input. Our AutoML™ process will customize the AI’s detection capabilities to your needs and improve your lab’s analysis workflow.
- Rewire Neuro and Neurescence are partnering to enhance fluorescence calcium imaging of the nervous system with cutting edge neuronal segmentation and analysis capability.
- Neurescence Inc. (Toronto, Canada), the manufacturer of the MultiscopesTM, and Rewire Neuro, Inc. (Portland, Oregon), a provider of artificial intelligence software development services (“AI-a-a-S”) announced their strategic development partnership today. With the aim to optimize the automated analysis workflow for neuroscientists, this technology integration utilizes deep learning to rapidly improve detection and quantification of neuronal activity while reducing the processing time of data-dense brain recordings. Videos that would previously take 2 weeks to be processed now result in neuronal detection in 30 minutes with double the accuracy compared to the existing automated neuronal segmentation methods in the market.
- RxHomeTest pitched to Inflect Health in January, 2022.
- Dr. Thomas R. Shearer discovered that the thinning of the retinal ganglion cell layer (GCL) occurs in patients with branch retinal artery occlusion (BRAO) as well as in central retinal artery occlusion (CRAO), recently published in PLosOne.
- We welcomed Takatoshi Uchida from Japan in 2022.
- In March 2022, Sirona Dx announced a co-marketing agreement with Ultivue for multiplexed tissue biomarker solutions.
- Sirona Dx announced a partnership with Scailyte for AI driven end-point specific single cell analysis in July 2022.
- Sonivate Medical, Inc. has received funding as part of an SBIR Program from the US Air Force for a Phase I ($74,886). It is a 90-day Phase I to be completed by January 27, 2023. This Phase I is to develop the requirements for an Ultra-High Frequency Linear Probe for clinical applications in MSK, ophthalmology and clinical ultrasound views < 3 cm.
- In addition, Sonivate will secure an Air Force clinician to be PI for the clinical research in the Phase II application.
Sparrow Pharmaceuticals (alumnus)
- Sparrow Pharmaceuticals received ethics committee and regulatory authority go-ahead for three Phase 2 clinical trials, in patients with Cushing syndrome, autonomous cortisol secretion, and polymyalgia rheumatica.
- Dr. David Katz was selected to give oral presentations on new clinical pharmacology and non-clinical study results at three prestigious conferences in May-June: European Congress of Endocrinology in Milan, European League Against Rheumatism in Copenhagen, and Endocrine Society in Atlanta.
- Sparrow has dosed the 1st patient in a phase 2 clinical trial of SPI-62 with prednisolone for polymyalgia rheumatica. Read press release here.
- Congratulations to Sparrow CSO and Founder, David Katz, for the honor of being a PharmaVoice 100! David was recognized for his entrepreneurial spirit and pursuit of finding a treatment for disorders due to steroid excess using scientific prowess and an artist’s vision. Read Dr. Katz’ interview.
- CSO and Founder Ken Stedman was interviewed by a local KATU reporter about the new SARS CoV-2 subvariant, which was then picked up and shown on Good Morning America.
- StoneStable, Inc. moved in to their new lab space in Suite 130A at the OBI.
- StoneStable, Inc. was one of the finalists for the Angel Oregon Life & Bio (AOBIO) investment. See press release.
- Ken Stedman and Dan Snyder from StoneStable were at the BIO meeting in San Diego and made a number of connections with international pharmaceutical companies, large and small.
- We are proud to announce that StoneStable, Inc. has been accepted into the Creative Destruction Lab program in the International Advanced Therapies Track.
- SUTUREGARD Medical, Inc. received the honor of being chosen by MedTech Innovator for the MedTech Innovator Road Show. In their words, ”MedTech Innovator is the largest and highest performing accelerator of medical technology in the world and the medtech industry’s premiere showcase and competition for innovative medical device, digital health and diagnostic companies.”
- We will be pitching April 14 and hope to leverage our participation into favorable business development pathways.
- We received a lot of attention at trade shows including the American College of Foot and Ankle Surgeons in Austin Texas where we presented our 3rd study showing ~80% reduction in postoperative wound complications after repair of ankle fractures.
- We have enjoyed record sales in Q1 2022.
- Dr Alton Johnson of University of Michigan Podiatry presented at the Society for Advanced Wound Care (SAWC) last month in Phoenix on HEMIGARD closure of Stage 4 (bone involved) heel wounds. He won first place in the Practice Innovation category for this work. HEMIGARD is allowing surgeons to close wounds that have not been able to be closed with normal suture materials. Normal suture does not work well on soft heel skin which does not resist tearing when under tension. HEMIGARD corrects this problem.
- The most recent episode (Season 4 – Episode 3 entitled “A Smell from Hell”) of “My Feet are Killing Me” featured HEMIGARD being used by “Dr. Brad” to close a very tough plantar wound. It does have graphic content including live surgery footage. The patient in question can hardly walk due to masses of callus on the ball of her foot – the surgical excision leaves a large wound that is difficult to close, and Dr. Brad uses HEMIGARD to help close the defect. The patient has an excellent result and is ecstatic! “My Feet Are Killing Me” A Smell from Hell (TV Episode 2022) – IMDb.
- Our biggest recent win had almost nothing to do with us. A general surgeon at Kaiser Permanente in Oakland got a hold of our wound closure device HEMIGARD, loved it, got it approved locally and then nationally and now we have received orders from multiple Kaiser clinical facilities. This surgeon is not an advisor or consultant to us, and just liked what HEMIGARD was doing for her wound closure results.
- We were one of 28 medical device startup companies selected from over 1000 worldwide applicants for the MedTech Innovator Accelerator 2022 program. This is wrapping up soon at AdvaMed in Boston at the end of the month and there are several competitions in play.
- One is the one minute video competition. Please enjoy (and “like” on LinkedIn) our story of how Dr. Brad used HEMIGARD to stop Shantell’s feet from killing her!
- SUTUREGARD wins American Diabetes Association 2022 Showcase Innovation Award. We were recently (November 4, 2022) honored to receive this award, having successfully competed with 160 other companies from the USA and beyond. Above we are receiving the award presented by Dr. Robert Gabbay, the Chief Medical Officer of the ADA (2nd from L). The ADA heard our message about how our HEMIGARD product has dramatically improved surgical outcomes for diabetic amputation patients.
- As you may know, many initial amputations do not heal on these patients who almost always have vascular disease and neuropathy, and often require more morbid above or below knee amputations with high risk of systemic complications including mortality. HEMIGARD reduced need for further amputation by 89% by preventing healing complications with the initial amputation. This study of 80 patients is valid real-world evidence that resonated with the ADA judges.
- We look forward to bringing HEMIGARD to all those who do provide surgical care for diabetics with the goal of improving their surgical outcomes!
- Synergic was a finalist in the 2022 Oregon Technology Awards in the Pre-Revenue category.
- Synergic is proud to announce that we have received breakthrough device designation from the FDA! This is a big day for us in our mission of bringing solutions to the Parkinson’s community. We appreciate this special recognition and support of the FDA in our on-going development of the VT Touch.
- Thaena held its annual 2022 Thaena Microbiome Symposium in a virtual format. It focused on the rise of probiotics: the roles they play in various organ systems, our health, and clinical applications.
- View recordings from the symposium here.
- Trace Biosciences (formerly Inherent Targeting) was selected as a finalist for the Angel Oregon Life & Bio (AOBIO) investment competition through OEN, competing for a ~$300k investment, with the winner announced at the Finale event on May 26th at Amaterra Winery in Portland. See press release.
- We were awarded an SBIR matching grant from Business Oregon for ~$50k to complete in vitro testing and regulatory planning of our lead contrast agent towards clinical translation.
- We were selected to participate in the Creative Destruction Lab Biomedical Engineering track taking place out of Vancouver WA.
- We received an SBIR matching grant from Business Oregon to complete some additional in vitro toxicology testing and regulatory work for our Phase I NSF STTR project.
- We received a phase I SBIR award from the NINDS at the NIH to develop our imaging agents for the application of intraoperative nerve damage assessment to improve nerve repair surgery.
- This fall, Trace Biosciences moved within the OBI to one of the new offices and a brand-new lab.
- Received a Business Oregon grant to help provide funding toward commercialization for its VT-101 oral medication for oncology patients.
- Founder and CEO Emmanuel Akporiaye, PhD, recently presented at an Angel Oregon Bio educational seminar.
- Their ongoing clinical trial for the study of Alpha-TEA and trastuzumab for the treatment of refractory HER2+ metastatic breast cancer continues at the University of Washington/Fred Hutchinson Cancer Center.
- Participated in the Research in Your Back Yard panel at the OBI in May.
- Recently enrolled a third patient in their clinical trial at Fred Hutchinson Cancer Center in Seattle and will soon be opening a second trial site at Providence Cancer Center, Portland.
- VIR Biotechnology graduated from the OBI February 28, 2022, after joining the incubator in 2015 as TomegaVax, an OHSU spinoff that is pursuing an HIV vaccine. VIR Biotechnology has had many successes throughout their time at the Oregon Bioscience Incubator, including initiating their Phase 1 clinical trial of VIR-1111, an investigational human immunodeficiency virus (HIV) T cell vaccine.
- Upon graduation, VIR Biotechnology has relocated their lab and office from OTRADI’s incubator in the Willamette Wharf building on Portland’s South Waterfront, to the Riverside Centre business park just down the street from the incubator.
- VBD was awarded its third year of funding on our SBIR Phase 2B grant focused on manufacture and toxicology of DRQ, our therapeutic for multiple sclerosis.
- After 20 years with Virogenomics and then with VBD, Jeff King has decided to step-down as CEO of VBD. He remains part of VBD’s Board of Directors. Renee Shirley will become CEO of VBD starting in 2023.
- VitalFlo officially joined the OBI in October 2022, moving into one of the newly built offices.
- VitalFlo helps leading clinicians and researchers improve patient outcomes and advance lung health by providing comprehensive respiratory data, analysis and care.
- VitalFlo Announces Public Investment Campaign to Help Doctors Measure, Monitor, and Predict Patient’s Lung Health
- “Do you think asthma and COPD should be treated preventatively instead of with trips to the ER? So does VitalFlo,” says Luke Marshall, PhD, VitalFlo Founder and CEO.
- The team at VitalFlo has built a spirometry platform to help patients and their primary care doctors proactively manage their asthma, COPD, and other respiratory concerns.
- VitalFlo has officially launched its Wefunder campaign. With Wefunder, everyone, regardless of background, connections, or income, will finally be able to invest in VitalFlo and be a critical part of their future success. Read More.
- VitalFlo was one of the finalists in the Impact category at Bend Venture Conference.
Congratulations to all of you on your hard work and successful endeavors! You’re doing great things for healthcare.
Business Oregon SBMA Grant
Do you have or know of a small business affected by COVID-19? Small businesses may be eligible for one of Oregon’s Small Business and Microenterprise Grant Assistance awards. This program is funded by CARES Act funding for businesses who meet eligibility requirements.
For more information, see Business Oregon’s SBMA Program page.
Note: Applications open on January 23 at 8 a.m. and are due at 5 p.m. on January 27, 2023.
Save the Date for OTRADI’s 15th Anniversary Celebration

Mark your calendars to come out and help us celebrate our 15th anniversary! More information to come, so stay tuned.
Thank You for Your Continued Support of our Programs

OBI is gearing up for some excellent free entrepreneurial programming this year. We have monthly Lunch & Learns on all kinds of interesting topics geared toward the life science community, monthly Happy Hour networking, a robust mentoring & advising program, and more. Each of these programs has sponsorship opportunities available.
To help support our programming with a tax-deductible sponsorship, please take a look at our Program Sponsorship Kit and contact Rene Miller for sponsorship or questions.
Upcoming OBI Events

OBI Virtual Lunch & Learn:
Diversity in Clinical Trials
January 18, 2023
12:00-1:00 PM
Register here
Accelerate Biotech + Digital Health Happy Hour
February 2, 2023
5:30-7:00 PM
Oregon Bioscience Incubator, Portland
Tickets are limited – get yours now!
OBI Virtual Lunch & Learn:
Business Oregon’s Commercialization Gap Fund
and SBIR Overview
February 28, 2023
12:00-1:00 PM
Save the date!
Other Community Events
PDX Women in Tech Presents:
January Happy Hour & Town Hall
January 17, 2023
5:30-7:30 PM
Virtual
Register here
UW WE-REACH Biomedical Entrepreneurship
Fireside Chat Series
January 19, 26, February 2, and 9, 2023
2:00-3:00 PM
Virtual
Register here
Lunch & Learn: What Every MedTech Startup Leader
Needs to Know About Attempting a Commercial Launch
January 25, 2023
12:00-1:30 PM
Virtual
Register here

Built Oregon Presents
PitchBlack 2023
February 7, 2023
6:30 PM
The Patricia Reser Center for the Arts, Beaverton
Tickets on sale now